compare: the elements
All known substances can be classified as solids, liquids, gases, or plasma. In addition, a fifth state of matter, the Bose-Einstein condensate has been discovered recently. However, it is not stable at normal earth conditions. Likewise, although plasma is the most abundant state of matter in the Universe, it is not common on the Earth under normal conditions, except for lightning. Most matter that students are familiar with will therefore be in a solid, liquid, or gaseous state.
An element is a pure substance that cannot be decomposed into simpler substances by normal chemical means. There are 109 different elements. Ninety of these are naturally occurring; the rest have been created in laboratories. Elements 110 and 118 are still being researched on. There will be more elements as technology can identify them. A symbol is used to represent the full name of an element. For example, H represents hydrogen; O represents oxygen, and Al represents aluminum. Sometimes the Latin name for an element is used as the basis for its symbol, for instance K represents potassium (kalium in Latin).
Three subatomic particles compose elements: protons, neutrons, and electrons. Protons, which have an electrical charge of +1, and neutrons, which have a neutral charge, make up the nucleus of an element. This nucleus is surrounded by a "cloud" of electrons, each of which as a charge of -1. The electrons spin around the nucleus in what are called orbits or shells. Each of the orbits can contain a set number of electrons. For instance, the first orbital from the nucleus has 2 electrons, the second has 8, the third has 8, the 4th has 16 and the fifth has 32, and so on. Each shell may not be full, depending on the number of electrons in the element, and the inner shells fill before the outer shells fill. Sodium, for example, has 11 electrons, which are located in the first, second, and third shells (2+8+1.)
An element has a uniform composition. Different elements may join together; these combinations are called compounds. A compound can be separated into its component elements by chemical means. For example, common table salt is a compound made of two elements: sodium and chlorine. Table salt can be broken down into sodium and chlorine by mixing it with water. However, sodium and chlorine cannot be easily broken down into any simpler forms.
PROCEDURE:
-
Discuss the properties of elements with the students. Review the structure of the periodic table. Ask students questions about the different elements and see if they can locate them on the periodic table.
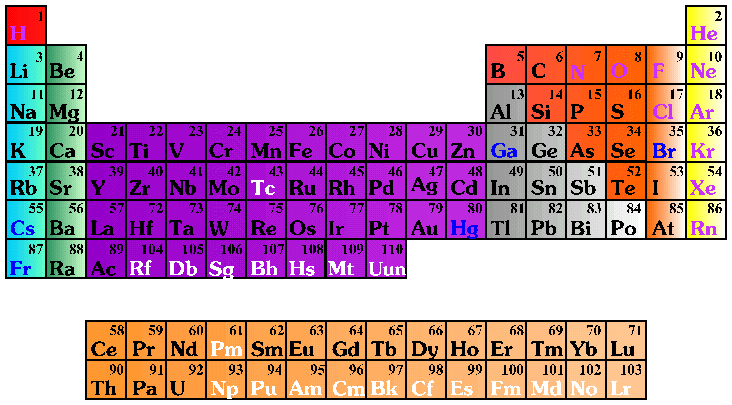
- Review the difference between an element and a compound. The students should realize that an element cannot be broken down, whereas a compound can be subdivided into elements. You may wish to explain that in many instances, forming or breaking down a compound requires energy. For example, if you place a mixture of iron and sulfur in a bowl, they will not react. No compound will form. However, if iron and sulfur are mixed and then heated, they will combine and form a compound.
- Write the following examples of compounds and their constituent elements on the board. At this point, do not be concerned with explaining the "endings" to the chemical words, such as chlorine versus chloride. These endings reflect the molecular structure of the compound.
ELEMENTS
COMPOUNDS
Na-sodium
NaCl (sodium chloride)
AgCl (silver chloride)
K-potassium
KCl (potassium chloride)
Ag-silver
KClO (potassium perchlorate)
O-oxygen
H2O (water)- Use the Periodic Table placemats to explore elements with the students. When they examine the chart, the students may ask the meaning of the numbers surrounding the element symbols. The number in the upper left corner is the atomic number, i.e., the number of protons inside the nucleus of the element. The number in the lower left is the atomic mass or atomic weight, which is essentially a measurement of how heavy the element is.
Explain the basic subatomic structure of elements. Tell the students that protons and neutrons reside inside the nucleus. The electrons spin around the nucleus in what are called orbits or shells. Each of the orbits represents a set number of electrons. For instance, the first orbit from the nucleus has 2 electrons, the second has 8, the third has 8, the 4th has 16 and the fifth has 32, and so on. Sodium for instance, has 11 electrons located in the first, second, and third shells (2+8+1.)
Elements
of Metal, Non Metal, Semi Metals
Known elements exist in the form of metals, not metals (non-metals), and semi metal.
1. Metal Elements
Metals are elements that have shiny properties and are generally good conductor of electricity and heat conductor. Metal elements are generally solid at normal temperatures and pressures, except for mercury in the form of liquid. In general, metal elements can be forged so that can be formed into other objects.
2. Non Metal Elements
The non-metallic element is an element which has no metallic properties. In general, non-metallic elements are gaseous and solid at normal temperature and pressure. Examples of non-metallic elements in the form of gases are oxygen, nitrogen, and helium. Examples of non-metallic elements in solid form are sulfur, carbon, phosphorus, and iodine. Non-metallic solids are usually hard and brittle. Non-metallic element in the form of liquid is bromine.
3. Semi Metal Elements
In addition to metal and nonmetal elements there is also a semilogam element or known as a metaloid. Metaloid is an element that has metallic and nonmetal properties. Semilogam element is usually semiconductor. What is a semiconductor? Semiconductor materials can not conduct electricity well at low temperatures, but their electrical conductivity becomes better when the temperature is higher.


BalasHapusIn the noble gas class, the element which has the greatest ionisation energy is ... ...?
In the class of noble gases, the element that has the greatest ionization energy is helium (He) because the noble gas ionization energy is getting lower it is getting smaller.
HapusCan you explain what happen if ozone was element?
BalasHapusOzone is formed in the atmosphere through several steps chemical processes that require the help of sunlight. In the stratospheric layer, the process of ozone formation begins with the breakup of oxygen molecules (O2) by ultraviolet radiation from the sun. In the lower atmosphere (troposphere), ozone is formed through a series of different chemical reactions and involves gases containing hydrocarbons and nitrogen.
HapusStratospheric ozone is naturally formed by chemical reactions involving solar ultraviolet radiation and oxygen molecules available in the atmosphere. Sunlight breaks down the oxygen molecule (O2) produces two oxygen atoms (2O). Then each of these oxygen atoms reacts with an oxygen molecule producing an ozone molecule (O3). The reaction occurs continuously due to the presence of solar ultraviolet radiation in the stratosphere. As a result, the largest ozone production occurs in the tropical stratosphere.
Reaction destruction / ozone destruction and the formation of O2 can take place via two paths:
O2 + O2 → 2O2
O3 + O3 → 3O2
This reaction is produced by a complex reaction with a gas and radical catalyst, such as Cl, NO, OH. The OH reaction can be formed by the destruction of H2O vapor, exhaust gases from supersonic aircraft. Cl radicals can be derived from chloroflurocarbons (CFCl or CFC-I I and CF2Cl or CFC-12) which are widely used in refrigerants and fuels (propellants).
Please give examples of commonly used elements in the lab
BalasHapusHydrogen chloride (HCl) is a monoprotic acid, which means that it can dissociate (ionize) release one H + (a single proton) only once. In a solution of hydrochloric acid, this H + combines with water molecules to form hydronium ions, H3O +: [22] [23]
HapusHCl + H2O → H3O + + Cl-
Another ion formed is a chloride ion, Cl-. Hydrochloric acid can therefore be used to make chloride salts, such as sodium chloride. Hydrochloric acid is a strong acid because it dissociates completely in water.
Monoprotic acid has one acid dissociation constant, Ka, which indicates the degree of dissociation of the substance in water. For strong acids such as HCl, Ka values are quite large. Several attempts at theoretical calculations have been made to calculate Ka HCl values. When a chloride salt such as NaCl is added to the HCl solution, it will not change the pH of the solution significantly. This indicates that Cl- is a very weak conjugate base and HCl fully dissociates in the solution. For a moderate-to-concentrated hydrochloric acid solution, the assumption that the molarity of H + equals the molarity (units of concentration) of HCl is good enough, with precision reaching four significant digits.
Of the six strong mineral acids in chemistry, hydrochloric acid is the hardest monoprotic acid undergoing a redox reaction. It is also the most harmless strong acid to be handled compared to other strong acids. Although acidic, it contains non-reactive and non-toxic chloride ions. Hydrochloric acid in moderate concentrations is stable enough to be stored and continues to maintain its concentration. For this reason, plus the fact that this acid is available in the form of pure reagents, hydrochloric acid is a very good acidic reagent.
Hydrochloric acid is the acid of choice in titration to determine the amount of base. Stronger acids will give better results because of the obvious endpoints. Azeotropic acid (about 20.2%) may be used as a primary standard in quantitative analysis, although its concentration depends on atmospheric pressure when it is made.
Hydrochloric acid is often used in chemical analysis to "digest" analytical samples. Concentrated hydrochloric acid dissolves many types of metals and produces metallic chlorides and hydrogen gas. It also reacts with basic compounds such as calcium carbonate and copper (II) oxide, producing the soluble chloride that can be analyzed.