Using english to calculate stoichiometry
Understanding Stoicymetry
Stoichiometric Definition and Stoichiometric Type - Stoichiometry is a chemical branch associated with a quantitative relationship that exists between reactants and products in chemical reactions. The reactant is a substance that participates in a chemical reaction, and also the product is a substance obtained as a result of a chemical reaction.
Stoichiometry
The stoichiometry depends on the fact that the elements
behave in a predictable or predictable way, as well as material that can
neither be created nor destroyed.
Therefore, when the elements are combined then produce a chemical reaction, something known and also specific that will occur and the reaction can be predicted by elements and also the number involved. Stoichiometry is the mathematics behind chemistry.
The stoichiometric calculations can find out how the elements and also the components diluted in a solution whose concentration is known react in the experimental conditions. The word "Stoichiometry" comes from the Greek word "stoicheion", meaning "element" and also "metron" means "size".
Therefore, when the elements are combined then produce a chemical reaction, something known and also specific that will occur and the reaction can be predicted by elements and also the number involved. Stoichiometry is the mathematics behind chemistry.
The stoichiometric calculations can find out how the elements and also the components diluted in a solution whose concentration is known react in the experimental conditions. The word "Stoichiometry" comes from the Greek word "stoicheion", meaning "element" and also "metron" means "size".
The laws governing Stoichiometry
The stoichiometry
relies on law is like fixed comparative law, double comparative law and also
the law of conservation of the masses.
• The law of mass conservation
Using the laws of physics is like the law of conservation of mass, which holds that the mass of the reactants is equal to the mass of the product, Stoichiometry is used to gather information about the amount of various elements used in a chemical reaction,
• Comparative law remains
This law states that the chemical compound (substance consisting of 2 (two) or more ages) which always contains the same proportion of an element (compound with one atom type) with mass.
• Law of multiple comparison
This law is one of the basic laws of stoichiometry, aside from the law of fixed comparison. Sometimes it is also called Dalton's law. It is said that, if 2 (two) elements form more than one compound between them, then the mass ratio of a second element which joins the fixed mass over the first element of both will have a ratio of a small sum of the whole.
• The law of mass conservation
Using the laws of physics is like the law of conservation of mass, which holds that the mass of the reactants is equal to the mass of the product, Stoichiometry is used to gather information about the amount of various elements used in a chemical reaction,
• Comparative law remains
This law states that the chemical compound (substance consisting of 2 (two) or more ages) which always contains the same proportion of an element (compound with one atom type) with mass.
• Law of multiple comparison
This law is one of the basic laws of stoichiometry, aside from the law of fixed comparison. Sometimes it is also called Dalton's law. It is said that, if 2 (two) elements form more than one compound between them, then the mass ratio of a second element which joins the fixed mass over the first element of both will have a ratio of a small sum of the whole.
Stoichiometric explanation
Subject to the above laws, such chemical reactions may combine
in a defined chemical ratio. The sum of each element must be the same
throughout the reaction. In a balanced chemical reaction, the relationship
between the amount of reactants and the product will usually form the ratio of
integers. For example, in a reaction that forms ammonia (NH3), exactly 1 (one)
nitrogen molecule (N2) reacts with 3 (three) hydrogen molecules (H2) to produce
2 molecules of NH3. It can be described as follows
N2 + 3H2 ---> 2NH3
Thus, the stoichiometry can be used to calculate the quantity as the amount of product that can be produced when the reactants are given and also the percentage of reactants made into a known product.
N2 + 3H2 ---> 2NH3
Thus, the stoichiometry can be used to calculate the quantity as the amount of product that can be produced when the reactants are given and also the percentage of reactants made into a known product.
Stoichiometric Type
• Reaction stoichiometry
The stoichiometry is often used to balance the chemical equations found in reaction stoichiometry. It depicts that the quantitative relationship between substances is caused because they participate in chemical reactions.
• Composition Stoichiometry
The stoichiometry of this composition explains the quantitative (mass) relationship between an element in a compound. For example, the stoichiometry of the composition represents the (mass) nitrogen with the joined hydrogen and becomes a complex ammonia. Ie 1 mole of nitrogen and also 3 moles of hydrogen in each of 2 moles of ammonia. Mole is the unit used in chemistry for the amount of substance.
• Gas Stoichiometry
The type of stoichiometry is related to a reaction involving a gas, in which the gas is at a known temperature, pressure and volume and can also be considered an ideal gas. For gas, the ideal volume ratio is equal to the ideal gas law, but the ratio of the single reaction mass must be calculated from the molecular mass of the reactant as well as the product, in which the molecular mass is the mass of one (1) molecule of the substance.
The ideal gas is a theoretical gas consisting of 1 (one) set of randomly moving, non-interacting particles that adhere to an ideal gas law. The ideal gas law is an ideal gas state equation. The ideal gas law equation is as follows:
"PV = nRT, where P is pressure, V is volume and also T is absolute temperature, n is gas mol and also R is universal gas constant".
• Reaction stoichiometry
The stoichiometry is often used to balance the chemical equations found in reaction stoichiometry. It depicts that the quantitative relationship between substances is caused because they participate in chemical reactions.
• Composition Stoichiometry
The stoichiometry of this composition explains the quantitative (mass) relationship between an element in a compound. For example, the stoichiometry of the composition represents the (mass) nitrogen with the joined hydrogen and becomes a complex ammonia. Ie 1 mole of nitrogen and also 3 moles of hydrogen in each of 2 moles of ammonia. Mole is the unit used in chemistry for the amount of substance.
• Gas Stoichiometry
The type of stoichiometry is related to a reaction involving a gas, in which the gas is at a known temperature, pressure and volume and can also be considered an ideal gas. For gas, the ideal volume ratio is equal to the ideal gas law, but the ratio of the single reaction mass must be calculated from the molecular mass of the reactant as well as the product, in which the molecular mass is the mass of one (1) molecule of the substance.
The ideal gas is a theoretical gas consisting of 1 (one) set of randomly moving, non-interacting particles that adhere to an ideal gas law. The ideal gas law is an ideal gas state equation. The ideal gas law equation is as follows:
"PV = nRT, where P is pressure, V is volume and also T is absolute temperature, n is gas mol and also R is universal gas constant".
Understanding stoichiometric ratio
A number of stoichiometry or also the ratio of reagents
(substances added to a system in order
to create a chemical reaction) is either the amount or the ratio of which,
assuming that the result of a reaction is completed on a basis,
Among others
are as follows:
1. All reagents are consumed
2. There is no deficit reagent
3. There is no residual residue
4. Reactions will only occur or are created on stoichiometric ratios
1. All reagents are consumed
2. There is no deficit reagent
3. There is no residual residue
4. Reactions will only occur or are created on stoichiometric ratios
Stoichiometry Formulas
The
quantitative study of the relative amounts of reactants and products in chemical
reactions is referred to as stoichiometry. Stoichiometric problems are
solved in just four simple steps which are as following:
- First step is the balance the equation
- Convert units of a given reactants and products into moles.
- This is followed by calculating the moles of substance yielded by the reaction using the mole ratio,
- The final step is to convert moles of desired substance to desired units.
Let's
suppose the following reaction of iron and oxygen that result in rust formation
for stoichiometry calculation:
Fe+O2→Fe2O3
As
mentioned, the first step in stoichiometric calculation is the balancing the
equation. As we know that reactants of a chemical equation are never destroyed
or lost and therefore, the products of a reaction exactly correspond to the
original reactants. Now, let’s balance the given equation:
Fe+O2→Fe2O3
According to
the equation, 1 iron (Fe) atom react with two oxygen (O) atoms to obtain 2 iron
atoms and 3 oxygen atoms; the reaction is not balanced.
4Fe+3O2→2Fe2O3
According to
the above mentioned balanced equation, 4 atoms of iron and 6 atoms of oxygen
(since 3 ×2 = 6 ) react to form 4 iron (since
2 × 2 = 4 ) and 6 oxygen (2 ×3 = 6). The atoms on both sides of the equation match. Now, convert the
given units to moles using conversion factors and then calculate the moles of
substance.
Stoichiometry Formulas List
Stoichiometry
formulas used for stoichiometry calculations are as follows:
- One mole (1 mol) of an element = 6.022 × 1023
- entities (the Avogadro Number).
Therefore, 1
mol of carbon-12 means there are 6.022 × 1023 carbon-12 atoms; 1 mol of H2O contains
6.022 × 1023 H2
O
molecules.
- Molar mass of an element = mass(g)/mole.
- Molar mass of an compound = Sum total of molar masses of atoms of elements in the formula
- Mole to mass stoichiometry formula: Mass (g) = no. of moles×no. of grams1 mol
- Mass to mole stoichiometry formula: No. of moles = Mass(g)× 1 molno of grams
- Moles to substance entity (atoms, molecules etc) formula: No. of entities = no. of moles× 6.022×1023 entities1 mol
- Substance entity to mole stoichiometry formula: No. of moles = no. of entities×1 mol6.022×1023 entities
- Mass percent of an element in a compound is calculated using molecular mass and chemical formula.
The formula
is as follows:
Mass % of
element X = atoms of X in formula×atomic mass of X (amu)molecular(orformula) mass of compound (amu) ×100
Since the
chemical formula provides information about moles of each element in a
compound, the mass percent of element X can be determined using the molar mass.
The stoichiometry formula is as follows
Mass % of
element X = moles of X in formula×molar mass of X (amu)mass of one mole of compound(amu)
×100.
Stoichiometry Formulas Mole to Mole
The mole to mole stoichiometry
formulas determine the molar relationships of reactants and products in a
chemical reaction using the balanced chemical equation which in turn is used to
calculate the moles of a product produced from the given quantities of the
reactants. It follows the law of conservation of mass which states that reactants
of a chemical equation are never destroyed or lost and therefore, the products
of a reaction exactly correspond to the original reactants. Using the law, the
given equation is balanced and the stoichiometric ratios of the reactants and
products; these ratios can be used as conversion factors for mole-to-mole
conversions.
Now, let’s
suppose, we want to calculate the number of mole produced by 2 moles of oxygen.
For the purpose, the following equation will be used
H2+O2→H2O
.
Now, let’s
first balance the equation. By balancing the equation, we get following one.
2H2+O2→H2O
.
Here, we can see one mole of oxygen produces two moles of water. The stoichiometric ratio is 1 mole of oxygen: 2 mole of water. Let’s assume that there is abundant hydrogen gas available for reaction. Under this condition,
2 mole of oxygen×2 mole of water1 mole of oxygen
= 4 moles of water
Mass to Mole Stoichiometry Formula
It uses molar
mass as a conversion ration. Molar mass refers to the mass of a given
substance divided by its amount (mol). As we know that mass of a substance is
calculated by measuring the amount of the substance and then converting it into
the moles. This is followed by calculating the substance's molar mass by
multiplying its relative atomic mass to the molar mass constant (1 g/mol).The
molar mass constant is then multiplied by the given mass to convert mass to
moles.
Example: For example, convert 20 grams of water to moles of water. The molar mass of water is 20 g/mol. Therefore the molecular weight of water in g/mol can be used to convert gram to mole. Thus,
Example: For example, convert 20 grams of water to moles of water. The molar mass of water is 20 g/mol. Therefore the molecular weight of water in g/mol can be used to convert gram to mole. Thus,
20 g water×1 mol20g water
= 1 mol water.
Grams to Grams Stoichiometry Formula
The grams to
grams stoichiometry formula converts mass of one substance into that of
other which cannot be done directly. Instead, it is done by converting the mass
of first substance into mole using mass to mole stoichiometry formula followed
by mole to mole conversion factors and then convert the mole of second
substance into mass. The first step is to use molar mass as conversion factor
to convert the mass of substance A into mole. The second step is to use mole
ratio as conversion factor to convert mole of substance A into that of
substance B. Third step is to use molar ratio to convert the mole of substance
B into mass of substance B.
Gas Stoichiometry Formula
According to
the “ideal gas law”, at temperature (T) in Kelvin, the volume (V) occupied
by n moles of any gas has a pressure (P).
The equation for gas law is given as following:
The equation for gas law is given as following:
P V = n R T.
Here R is known as the gas constant and the equation is called the ideal gas law or equation of state. Under these conditions, 1 mole of gas occupies 22.4 litres of volume. The ideal gas law and balanced gas equations are used for stoichiometry calculations of gaseous reactions using gas stoichiometry formula

Hydrogen gas can be made from the reaction of Zinc metal with a solution of sulfuric acid. Calculate the 2 M sulfuric acid volume required to produce 6.72 liters of hydrogen gas (STP).
BalasHapusResolution:
HapusZn + H₂SO₄ ---> ZnSO₄ + H₂
2M 6.72 L
NH₂ = VH₂ / VSTP
= 6.72 L / 22.4 L
= 0.3 mol
NH₂SO₄ = Coefficient H₂SO₄ / Coefficient H₂ × nH₂
= 1/1 × 0.3 mol
= 0.3 mol
N = M × V
V = n / M
= 0.3 mol / 2
= 0.15 L
How many grams of water vapor mass of 5.6 liters at STP (Ar H = 1, O = 16) ?
BalasHapusWater vapor = H₂O
HapusMr H₂O (relative period) = 2 + 16 = 18
Mass H₂O = Volume HOO (Liter) / 22.4 × Mr HOO
Thus, Mass H₂O = 5.6 Liter / 22.4 liters × 18 = 4.5 grams.
What is the strong evidence or reason in your article that states that the mass of the reactants equals the mass of products in mass conservation law?
BalasHapusThe law of conservation of mass or otherwise known as Lomonosov-Lavoisier law is a law that states the mass of a closed system will be constant despite the various processes within the system (in a closed system The mass of substances before and after the reaction is the same (constant)) . The commonly used statement to express the law of conservation of mass is that mass can be deformed but can not be created or destroyed. For a chemical process within a closed system, the mass of the reactants must be equal to the mass of the product.
HapusThe law of conservation of mass is widely used in fields such as chemistry, chemical engineering, mechanics, and fluid dynamics. Based on the special science of relativity, mass conservation is a statement of the conservation of energy. The fixed particle mass in a system is equivalent to its central momentum energy. In some radiation events, it is said that the mass changes to energy. This happens when an object changes into kinetic energy / potential energy and vice versa. Since mass and energy are related, in a system that gets / releases energy, a very small amount of mass will be created / lost from the system. However, in almost all events involving changes in energy, mass conservation laws can be used because the changed mass is very small.
How gr mass of each? elements contained in 15.5 grams of calcium phosphate. Ca3 (PO4) 2?
BalasHapusCa mass in Ca3 (Po4) 2 = (number of N atoms (Ar N)) / (MrCa3 (PO4) 3) x 15.5 g
Hapus= ((3) (40)) / 310 x 15.5 g
= 6 g
Mass P in Ca3 (Po4) 2 = (number of atoms P (Ar P)) / (MrCa3 (PO4) 3) x 15.5 g
= ((2) (31)) / 310 x 15.5 g
3.1 g
Mass O in Ca3 (Po4) 2 = (number of O atoms (Ar O)) / (MrCa3 (PO4) 3) x 15.5 g
= ((2x4) (16)) / 310 x 15.5 g
= 6.4 g
Ca mass in Ca3 (Po4) 2 = (number of N atoms (Ar N)) / (MrCa3 (PO4) 3) x 15.5 g
= ((3) (40)) / 310 x 15.5 g
= 6 g
Mass P in Ca3 (Po4) 2 = (number of atoms P (Ar P)) / (MrCa3 (PO4) 3) x 15.5 g
= ((2) (31)) / 310 x 15.5 g
3.1 g
Mass O in Ca3 (Po4) 2 = (number of O atoms (Ar O)) / (MrCa3 (PO4) 3) x 15.5 g
= ((2x4) (16)) / 310 x 15.5 g
= 6.4 g
PV = nRT
BalasHapuswhen we use this formula??
And can you give me the example?
Before experimation better first acquaintance ideal gas deh. The ideal gas is a gas that works at room pressure and temperature. This ideal gas formula can be determined by the following general formula:
HapusPV = nRT
P = Pressure (atm / Pa)
V = Volume (L)
N = Number of Moles (mole)
R = Fixed (0.082 or 8314)
T = Temp (K)
From the above formula we can conclude with this statement as follows:
The pressure is directly proportional to the temperature
This means that every temperature in the rise, then automatically tekana will mingkat
The pressure is inverted with volume
Artinnya any objects that the volume in the enlarged the pressure will decrease
Sebeliknya when the volume on the smaller causes greater pressure
The pressure is directly proportional to the number of moles
This means that if the number of moles of a substance in the feed in a fixed volume container, it can increase the pressure inside the container
Does gay lussac law apply to each reaction?
BalasHapus
HapusNo, since this law applies only to The ratio of volumes between gases in a chemical reaction is a simple integer ratio and the pressure of a fixed amount of gas at a volume that remains proportional to its temperature in the kelvin.
A total of 20 ml of HCl solution was titrated by a 0.1 M NaOH solution using a phenolphthalein indicator. If a pink color change takes 25ml of penetration solution, then how many molecules of HCl solution?
BalasHapusNormalities of NaOH solution, Nb = Mb = 0.1 M
HapusVa x Na = Vb x Nb
20 x Na = 25 x 0.1
Na = 0.125 N
So kemolaran HCL = Na = 0.125 M
Why and how to calculate mass percentage with pressure, temperature, and volume?
BalasHapusOne mole of the substance represents the number of substances containing the number of particles equal to the number of particles in 12.0 grams of C-12 isotope.
HapusFor example:
1. 1 mol of Na element contains 6.02 x 1023 Na atoms.
2.1 mol of water compound contains 6.02 x 1023 water molecules.
3. 1 mol of NaCl ion compound contains 6.02 x 1023 Na + ions and 6.02 x 1023 Cl- ions.
Relation of Moles to Number of Particles
The mole relationship with the number of particles can be formulated:
Quantity (in moles) = number of particles / NA
or
Number of particles = mol x NA
Problems example:
A sample contains 1.505 x 1023 molecule Cl2, how many moles of Cl2 content is it?
Answer:
Quantity (in mol) Cl2 = number of Cl2 / NA particles
= 1.505 x 1023 / 6.02 x 1023
= 0.25 mol
Relation of Moles to Mass
Before discussing the relationship of moles with the masses, you must first remember about Relative Atomic Mass (Ar) and Relative Molecular Mass (Mr). Still remember? Then we check your memories by doing the following.
Calculate Mr. H2SO4 (Ar H = 1, S = 32, and O = 16)!
Given the relative atomic mass (Ar) of several elements as follows.
Ca = 40
O = 16
H = 1
Determine the relative molecular mass (Mr) of Ca (OH) 2!
Do you remember? Then we go directly to the next matter that is about the molar mass.
The molar mass represents the mass possessed by 1 mol of substance, equal to the Ar or Mr.
For element:
1 mol element = Ar gram, then it can be formulated:
Mass 1 mol of substance = Ar substance expressed in grams
or
The molar mass of the substance = large Ar of gram / mol
For compounds:
1 mol of compound = Mr gram, then it can be formulated:
The mass of 1mol substances = Mr substances expressed in grams
or
The molar mass of the substance = large Mr. gram / mol substance
Thus the difference between the molar mass and the relative molecular mass is in the units. The molar mass has a gram / mol unit while the relative molecular mass has no units.
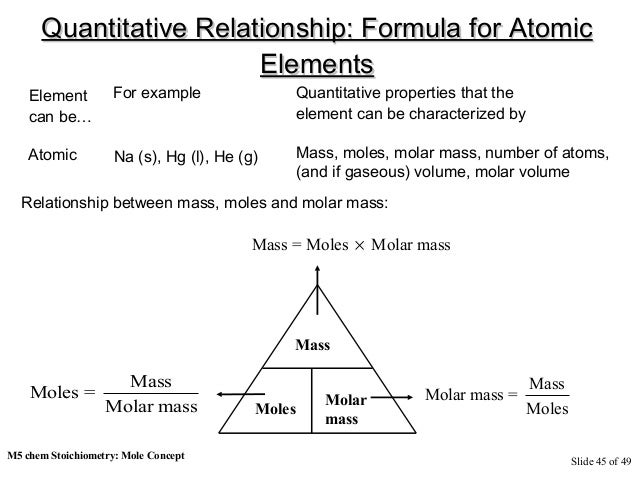
The relationship between mol and mass is:
Quantity (in mol) = Mass of compound or element (gram) / Molar mass of compound or element (gram / mol)
Mole Relations with Volume
A. Gas in the standard state
Measurement of gas quantity depends on temperature and gas pressure. If the gas is measured in a standard state, then the volume is called the molar volume. The molar volume is the volume of 1 mole of gas measured under standard circumstances. The standard state is at 0 ° C (or 273 K) and atmospheric pressure (or 76 cmHg or 760 mmHg) or STP (Standard Temperature and Pressure).
The amount of gas molar volume can be determined by the ideal gas equation: PV = nRT
P = pressure = 1 atm
N = mol = 1 mole of gas
T = temperature in Kelvin = 273 K
R = gas constant = 0.082 liter atm / mol K
Then:
P V = nRT
V = 1 x 0.082 x 273
V = 22,389
V = 22.4 liters
Thus, the standard volume = VSTP = 22.4 Liter / mol.
Can be formulated: V = n x Vm
N = number of moles
Vm = VSTP = molar volume
Can you please tell how to get the volume constant of STP 22,4 ??
BalasHapusConditions with 0 ° C and 1 atm pressure are called standard states and are expressed by STP (Standard Temperature and Pressure).
HapusPV = nRT
with:
P = pressure (atm)
V = volume of gas (liter)
N = number of moles (mole)
R = gas constant = 0.082 L atm / mol K
V = nRT/P
V = (1 mol ×0,082 Latm / mol K ×273K)/1atm
V = 22,4 liter
Thus, in the standard state (STP), the molar volume (volume 1 mole of gas) is 22.4 liters / mol.